Periapical Microsurgery: To Graft or Not To Graft …
By Peter Z. Tawil, DMD, MS, and Jackson T. Seagroves, DDS, MS
Introduction
Periradicular Microsurgery is an important treatment option in modern endodontic practices and is a crucial asset for those practitioners that embrace it (1). The “Renaissance Era of Microsurgical Endodontics” has occurred with endodontists at the forefront, making our specialty shine. Although dental implants remain a great option for replacement of the “missing and hopeless” dentition, long-term data on dental implants have highlighted their limitations (2) and therefore implant surgeons are looking to maintain the natural dentition for as long as possible. As a result we are now being asked to save more and more teeth by clinicians who are familiar with, and often expect, various grafting and guided tissue regeneration protocols.
Modern microsurgical endodontic protocols (3) in conjunction with contemporary root-end filling materials have yielded excellent success rates and predictability (4-6). Despite these advancements there is still disagreement and outright confusion regarding the role of grafting. To Graft or Not To Graft … that is the outstanding question that many of us are being asked by our implant-focused colleagues who graft routinely for their therapies, who often wonder, “Why are you leaving a hole in the bone?”. Their rationale in utilizing grafting protocols is to facilitate osseous healing and to assist in the reconstruction of the compromised bony housing, however there is little agreement among endodontists regarding the benefit of grafting in conjunction with periradicular surgery.
The lack of consensus and overall confusion in our specialty is expected with a dearth of high-quality, prospective, longitudinal clinical research on the effect of grafting on the success of periradicular surgery (7). This topic was reviewed expertly by von Arx in 2011, who highlighted problems with the evidential basis of grafting post-apical microsurgery, namely the scarcity of studies combined with low generalizability due to small sample sizes, lack of controls, and improper study design. These methodological concerns present over a decade ago are still relevant today and serve to complicate our understanding and acceptance of grafting protocols. At a high level, grafting and guided tissue regeneration techniques are based on differentiation and proliferation of desired cells (osteoblasts, cementoblasts, PDL fibroblasts) with the exclusion of undesirable cells (gingival fibroblasts) in the osseous defect in order to form new tissues with structure and function identical to the missing tissue (8). Today there is an abundance of bone replacement options, membranes, and growth factors available on the market. There are many industry-driven courses, sponsored speakers, and online influencers muddying the waters by recommending various products with little research support. If we are astute, evidence-based clinicians and take a critical look at the studies quoted by these various entities, one will likely return to the same conclusion Von Arx came to in 2011, which we will review below (7).
Review
Before we review this topic we must start by defining the various type of lesions encountered in periradicular surgery:
- Type I Apical Lesion: An isolated endodontic lesion surrounded by bone with possible partial erosion of one cortical plate.
- Type II Through and Through Lesion: A tunnel-type lesion with loss of both buccal and lingual cortical plate This may be a lesion eroding both cortical plates or it may occur when a buccal surgical approach is needed to address a lingual lesion resulting in a loss of both the buccal and lingual cortical plates.
- Type III Endo-Perio Lesion: An extensive lesion with a complete loss of cortical plate including cervical bone creating an apico-marginal defect.
Type I Apical Lesion
Type I apical lesions are 5-walled defects which present an ideal biological environment for bone regeneration after addressing the microbial etiology from the canal system. Endogenous growth factors are released by immune cells and the surrounding endosteum to facilitate healing in the absence of a grafting material (9). Although many lesions appear completely healed when assessed with two-dimensional periapical radiography, there is a lag in healing when assessed with three-dimensional imaging, particularly concerning regeneration of the cortical plate. Using CBCT to measure healing of Type I apical lesions, Crossen (2019) found a 0.25mm reduction in buccal cortical plate thickness at 12 months after periapical microsurgery without grafting (9). von Arx highlighted a similar “cupping” effect in his 1- and 5-year CBCT reviews of periapical healing, finding improvement in apical bone at the resection plane and within the former apical defect while the buccal cortical bone significantly lagged behind (10). The effect of lesion size on expected healing described by von Arx is in general agreement with previous studies showing improved healing of Type I apical lesions exceeding 10mm in diameter when grafting is utilized (11-14).
Type II Through & Through Lesion
Type II through & through lesions consist of loss of both buccal and lingual cortical plates. The lesion may be a true tunnel-type lesion due to apical periodontitis affecting both cortical plates, or it can occur iatrogenically from a buccal surgical approach to a lingual lesion. These cases are characterized by a 3- or 4-walled bony crypt and may fail to completely heal due to altered healing dynamics with an absent periosteum and endosteum. Deficiency in these tissues may limit the osteoblastic repopulation of the defect, which may be outpaced by proliferation of gingival fibroblasts leading to an ingrowth of connective tissue. This incomplete healing pattern of Type II lesions with scar was described classically by Molven 1996 (15). Although histologically free of inflammation, persistent apical rarefactions can result in mis-diagnosis and overtreatment with the increased utilization of 3D radiography and a mobile society (16). The fibrous tissue bridge may also complicate osseointegration of a future implant in the event the tooth is extracted due to fracture or restorative considerations. While studies describing grafting in Type II lesions are limited by study design and statistical power, many do show a benefit to grafting with reduced scar tissue formation. Pecora (2001), and Murashima (2002) found a clinical and histological benefit of grafting Type II lesions with calcium sulfate, while Taschieri (2007) showed promising results with a bone particulate and membrane technique (12, 18, 17).
Type III Endo-Perio Lesion
Type III endo-perio lesions consist of apical and cervical bone loss that merge to form a continuous apico-marginal defect. These lesions have a 2- or 3-walled bony crypt with a complete lack of bone along the buccal surface of the root. Without a guided tissue regeneration technique, gingival epithelium may migrate along the denuded root surface faster than other progenitor cells from bone, cementum, or PDL, resulting in formation of long junctional epithelium and an increased risk of gingival recession post-operatively. This pattern of healing may be a significant esthetic concern and may also impact success and survival of teeth after periapical surgery (19).
Although the rationale for grafting in Type III lesions is biologically sound and clinically apparent, there is little consensus in the literature regarding its importance or ideal technique. Many studies have shown favorable outcomes with a variety of grafting protocols using bone particulate and collagen membrane (Dietrich 2003), periosteal sliding graft and polyglactin membrane (Marin 2006), and calcium sulfate and collagen tape (Kim 2008) (20-22). However, the generalizability of these findings is limited by lack of control groups and standardization of outcome measures. One of the few randomized controlled trials with adequate controls and outcome standardization (Dhiman 2015) compared platelet-rich fibrin (PRF) grafts with no graft in Type III lesions, finding comparable success rates of 86.66% and 80%, respectively (23). At 1 year, teeth with PRF grafts had a significantly greater probing depth reduction, with non-significant improvements in periapical healing and clinical attachment level (23). Given the limited high-quality research in this area, prudent clinicians should approach these cases with caution and discuss with patients the variable outcome of apical microsurgery in teeth with extensive periodontal involvement.
Materials & Techniques
Grafting is a regenerative therapy that aims to stimulate osteogenesis while precluding proliferation of undesirable tissues (e.g. epithelium or connective tissue) in the bony crypt created due to apical periodontitis and associated surgical intervention. Materials for grafting may contain bone-forming cells (osteogenic) or growth factors to stimulate host cells to produce bone (osteoinductive), or may serve as a physical scaffold for deposition of new bone (osteoconductive). They are classified based on their source and constituents which inform their properties of osteogenicity, osteoinductivity, and osteoconductivity.
Autograft
Autografts are bone grafts sourced from the same patient. Although it is an ideal material given its osteogenic, osteoinductive, and osteoconductive potential, it is not practical as it requires a second surgical site to harvest.
Allograft
Allografts are sourced from human cadavers in the form of freeze-dried bone or decalcified freeze-dried bone. They have both osteoinductive and osteoconductive properties.
Alloplast
Alloplastic grafts are a heterogenous group of materials that include calcium sulfate, calcium phosphate, hydroxyapatite, calcium carbonate, bioactive glass, and various polymers. The most studied alloplastic graft in apical microsurgery is calcium sulfate, which has shown favorable clinical and histological results (12,18). Alloplastic grafts mainly serve as a scaffold and are therefore osteoconductive, however some materials incorporate biologics that increase their osteoinductivity.
Xenograft
Xenografts are bone grafts harvested from a non-human source, typically from a bovine donor. They have osteoconductive properties, but may have different physical and chemical characteristics compared to human bone. There are also concerns about possible disease transmission and immunogenic potential (24).
Application of these various materials may mirror the cross-sectional anatomy of the area to be regenerated, dividing grafts into cancellous bone replacement materials and cortical bone replacement materials.
Cancellous bone replacement materials
Cancellous bone replacement materials should ideally have a small particle size and be relatively permeable to facilitate movement of blood for wound healing and osteogenesis to occur. Many support the use of allografts for this indication given their osteoinductive and osteoconductive properties. They have a variable particle size that mimics the radiographic appearance of bone. Earlier studies focused on alloplastic grafts such as calcium sulfate whose original paste formulation had a hard set, precluding ideal blood flow. More recently, particulate forms of alloplastic materials including calcium sulfate, hydroxyapatite, and tricalcium phosphate have been introduced with more favorable osteoconductive properties. Xenografts such as collagen plugs may play a minor role in healing of small defects, and certainly are useful to seal an oro-antral communication in the event of a sinus exposure (7).
Cortical bone replacement materials
Cortical bone replacement materials function to prevent the ingrowth of soft tissues into the crypt, allowing time for the bony matrix to form. An autograft may be useful in situations with thick cortical bone. In the “Bony Lid” technique, a piezotome is used to harvest a window of buccal bone, which is replaced after apical surgery and secured with an alloplastic calcium sulfate graft (25). Allograft and xenograft membranes can also be used, but in large defects it is critical to place a cancellous bone material to support and prevent collapse of the non-rigid membrane into the defect. Non-resorbable alloplastic materials such as ePTFE have been used historically, and more modern alloplastic grafts have resorbable properties to avoid a second surgery. Similar to the “Bony Lid” technique, a calcium sulfate alloplastic graft may also be used to reform the cortical plate and effectively seal the cancellous bone replacement material (see figures).
Conclusion
Although grafting is not always needed in periapical microsurgery (8), evidence-based clinicians will likely return to the same conclusion von Arx reached in 2011 which shows benefit with periodontal defects, through-and-through lesions, and lesions greater than 10mm in diameter (7). It is important to note that despite their proposed benefits, radiopaque grafting materials may confound our traditional radiographic assessment of periapical healing. The potential biological benefits provided by grafts do warrant consideration of a mindset shift in healing assessment from the strict measures “success” or “failure” we are accustomed to, particularly in the age of 3D radiography. A grafting mindset can help maximize the healing potential and avoid scar tissue formation, which may prevent misdiagnosis and overtreatment particularly with an increasingly mobile population (16). In a time when many teeth that could be saved are replaced with an implant, it’s up to the specialists dedicated to “saving the natural dentition” to embrace microsurgical endodontics and grafting techniques when appropriate.
References
- Boykin MJ, Gilbert GH, Tilashalski KR, Shelton BJ. Incidence of endodontic treatment: a 48-month prospective study. J Endod 2003 Dec;29:806-809.
- De Kok IJ, Duqum IS, Katz LH, Copper LF. Management of Implant/Prosthodontic Complications. Dent Clin North Am 2019 Apr;63:217-231.
- Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod 2006 Jul;32:601-623.
- Rubinstein RA, Kim S. Long-term follow-up of cases considered healed one year after apical microsurgery. J Endod 2002 May;28:378-383.
- Chong BS, Pitt Ford TR, Hudson MB. A prospective clinical study of Mineral Trioxide Aggregate and IRM when used as root-end filling materials in endodontic surgery. Int Endod J 2003 Aug;36:520-526.
- Kohli MR, Berenji H, Setzer FC, Lee SM, Karabucak B. Outcome of Endodontic Surgery: A Meta-analysis of the Literature-Part 3: Comparison of Endodontic Microsurgical Techniques with 2 Different Root-end Filling Materials. J Endod 2018;44:923-931.
- Von Arx T, AlSaeed M. The use of regenerative techniques in apical surgery: A literature review. Saudi Dent J, 2011, 23:113-127.
- Bashutski J, Wang H. Periodontal and endodontic regeneration. J. Endod. 2009;35:321–328.
- Crossen D, Tawil P, Morelli T, Tyndall D. Periapical Microsurgery: A 4-D Analysis of Healing Patterns. J Endod, 2019, 45:402-5.
- Von Arx T, Janner S, Hänni S, Bornstein M. Radiographic Assessment of Bone Healing Using Cone-Beam Computed Tomographic Scans 1 and 5 Years after Apical Surgery. J Endod, 2019, 45:1307-13.
- Pecora, G., Kim, S., Celletti, R. & Davarpanah, M. (1995) The guided tissue regeneration principle in endodontic surgery: one-year postoperative results of large periapical lesions. International Endodontic Journal, 28, 41–46.
- Pecora, G., De Leonardis, D., Ibrahim, N., Bovi, M. & Cornelini, R. (2001) The use of calcium sulphate in the surgical treatment of a ‘through and through’ periradicular lesion. International Endodontic Journal, 34, 189–197.
- Rankow, H.J. & Krasner, P.R. (1996) Endodontic applications of guided tissue regeneration in endodontic surgery. Journal of Endodontics, 22, 34–43.
- Tobon, S.I., Arismendi, J.A., Marin, M.L., Mesa, A.L. & Valencia, J.A. (2002) Comparison between a conventional technique and two bone regeneration techniques in periradicular surgery. International Endodontic Journal, 35, 635–641.
- Molven O, Halse A, Grung B. Incomplete healing (scar tissue) after periapcial surgery — radiographic findings 8 to 12 years after treatment. J Endod, 1996, 22:264-8.
- Kruse C, Spin-Neto R, Reibel J, Wenzel A, Kirkevang L. Diagnostic validity of periapical radiography and CBCT for assessing periapical lesions that persist after endodontics surgery. Dentomaxillofac Radio. 2017;46(7)
- Taschieri S., del Fabbro M., Testori T., Weinstein R. Efficacy of xenogeneic bone grafting with guided tissue regeneration in the management of bone defects after surgical endodontics. J. Oral Maxillofac. Surg. 2007;65:1121–1127.
- Murashima Y., Yoshikawa G., Wadachi R., Sawada N., Suda H. Calcium sulfate as a bone substitute for various osseous defects in conjunction with apicectomy. Int. Endod. J. 2002;35:768–774.
- Skoglund, A., Persson, G., 1985. A follow-up study of apicoectomized teeth with total loss of the buccal bone plate. Oral Surg. Oral Med. Oral Pathol. 59, 78–81.
- Dietrich T., Zunker P., Dietrich D., Bernimoulin J.P. Periapical and periodontal healing after osseous grafting and guided tissue regeneration treatment of apicomarginal defects in periradicular surgery: results after 12 months. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003;95:474–482.
- Marin M.L., Dominguez J.S., Arismendi J.A., Mesa A.L., Florez G.A., Tobon S.I. Healing response of apicomarginal defects to two guided tissue regeneration techniques in periradicular surgery: a double-blind, randomized clinical trial. Int. Endod. J. 2006;39:368–377.
- Dhiman M., Kumar S., Duhan J., Sangwan P. , MDS, Tewari S. Effect of Platelet-rich Fibrin on Healing of Apicomarginal Defects: A Randomized Controlled Trial. J Endod, 2015, 31:985-91.
- Kim E., Song J., Jung I., Lee S., Kim S. Prospective clinical study evaluating endodontic microsurgery outcomes for cases with lesions of endodontic origin compared with cases with lesions of combined periodontal-endodontic origin. J Endod, 2008, 34:546-51.
- Titsinides S, Agrogiannis G, Karatzas T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jon Dent Sci Rev, 2019, 55:26-32.
- Niemczyk S., Barnett F., Johnson J., Ordinola-Zapata R., Glinianska A., Bair J., JangA. PRESS and Piezo Microsurgery (Bony Lid): A 7-Year Evolution in a Residency Program Part 1: Surgeon-defined Site Location. Journal of Endodontics, 2022, 48, 787-96.
Figures
Case 1: Type I Apical lesion with over 10mm wide defect.
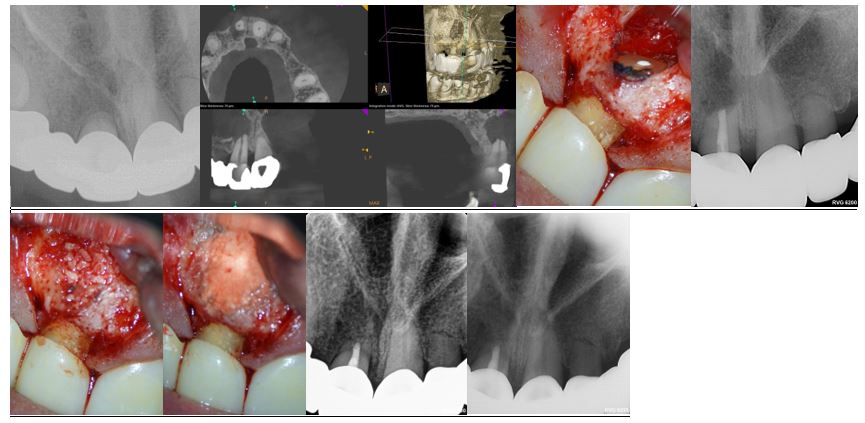
Preop, Preop CBCT, Root end fill image, Root end fill bioceramic, Allopast Mineralized 50/50 replacing the cancellous bone, Calcium sulfate replacing the cortical plate, post op, 1 year follow up.
Case 2: Type 3 Apical lesion with over 10mm wide defect & Endo-Perio component.
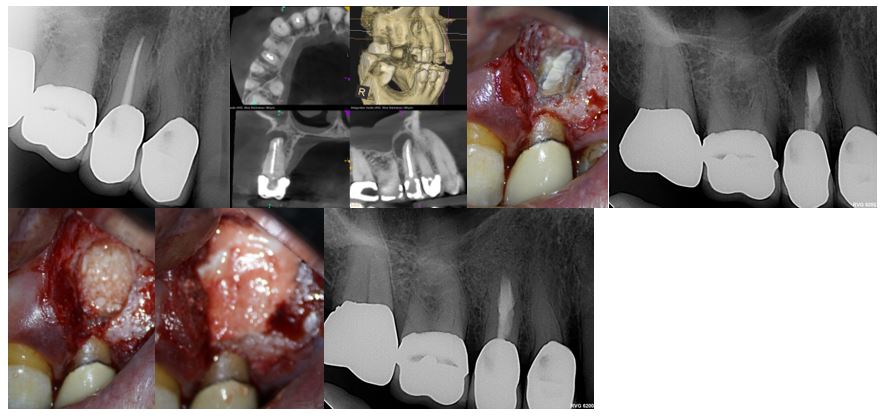
Preop, Preop CBCT, Root end fill image, Root end fill bioceramic, Allopast Mineralized 50/50 replacing the cancellous bone, Calcium sulfate replacing the cortical plate, post op.
