A Concise Guide to Pathology within Endodontics
 By Gabriella Blazquez
By Gabriella Blazquez
If the average endodontist sees just four cases per workday, they will have encountered well over thirty thousand patients throughout their career. The vast majority of periapical pathology seen in these patients will meet the criteria of apical periodontitis1. Still, it is important to be aware of other, less common, pathological entities which can and will present in such a large pool of endodontic patients. This paper seeks to outline the distinguishing features of apical periodontitis and other forms of periapical pathology. It is my hope that this article will serve as a brief review of relevant pathologies for endodontists, endodontic residents, and predoctoral students interested in the field.
Apical periodontitis is the most frequently encountered and therefore the most pertinent pathological process within endodontology. In the vast majority of cases, it occurs when microbes from deep carious lesions infiltrate the dental pulp and establish an infection. In some instances, apical periodontitis can be initiated by trauma. The requirement of microbes to initiate apical periodontitis has been demonstrated by Kakehashi et al.2 The resulting inflammation builds up within the enclosed pulpal space and tends to leach out of the apical foramen and into the surrounding periodontium. There, microbial and necrotic debris initiate an inflammatory cascade, which ultimately leads to soft tissue and alveolar bone destruction, visible radiographically as a periapical radiolucency.3
This apical periodontal inflammation can sometimes resolves on its own, but in many cases it leads to the formation of a periapical granuloma, cyst, abscess, or scar.
Each of these diagnoses describes a biologically distinct process, however, they are radiographically indistinguishable4. Notably, if a sinus tract forms and pus is clinically detectable, one can almost be certain that the radiographic lucency in question is a periapical abscess. However, the gold standard for diagnosing these conditions remains histological examination1. Without a biopsy, a clinician can only provisionally diagnose a well corticated radiolucent lesion surrounding the apex of a tooth with pulpal necrosis as apical periodontitis.
Various inflammatory mediators present at the apex of a necrotic pulp can initiate hyperplasia of granulation tissue, a fibro-vascular matrix which forms a scaffold to wall off an active periapical inflammatory process5. Periapical granulomas are surrounded by a fibrous capsule within which exists granulation tissue including new capillary loops, macrophages, plasma cells, as well as fibroblast cells, which produce collagen fibers6. In 2009, Schulz determined that 70% of periapical lesions obtained during apical surgeries could be diagnosed histologically as granulomas7.
Less frequently, about 23% of the time, samples taken from apical surgeries included periapical cysts7. These odontogenic inflammatory cysts are pathologic cavities whose lumens are lined by epithelium and a wall of connective tissue6. Periapical cysts form when inflammatory mediators at the apices of non-vital teeth stimulate cell rests of Malassez to proliferate and form the cyst’s epithelial lining8.
Rarer still were the periapical abscesses and scars, which made up just 5% and 1% of apical surgery biopsy samples, respectively7. Abscesses are localized collections of purulence, made up of white blood cells called PMNs, which often present as fluctuant nodules that express pus when manipulated. Periapical abscesses represent acute inflammatory exacerbations of apical periodontitis1. Finally, periapical scars represent a reparative process where fibrous connective tissue, specifically dense collagen, colonizes the periapical area.9
While the varied forms of apical periodontitis present as well-corticated periapical radiolucencies, our next pathology, known as condensing osteitis, appears radiographically as a well-localized opacity. Condensing osteitis is the most common radiopaque lesion of the jaws, which occurs as a result of chronic low-grade inflammation, and is most often seen in the periapical region of mandibular premolars and molars with pulpal necrosis.10
Odontogenic keratocysts (OKCs) are the most common odontogenic non-inflammatory lesions, making up 2.08% of periapical lesions submitted for biopsy.1 The term odontogenic means arising from tissues which make up teeth. OKCs most often present as a slow-growing, painless swellings of the posterior jaws. On radiograph, they appear as well corticated unilocular or multilocular lucencies. They can be destructive, tend to recur when removed, and are occasionally part of Basal Cell Nevus syndrome, which is also known as Gorlin-Goltz syndrome. This syndrome classically presents in the first thirty years of life with multiple odontogenic keratocysts, basal cell carcinomas, and bifid ribs.11
Ameloblastomas are benign odontogenic neoplasms of epithelial origin which make up 1% of oral tumors12 and 0.3% of all periapical biopsies.1 Despite their benign status, ameloblastomas are unencapsulated, aggressive, destructive, and locally infiltrative. They also have high recurrence rates when removed conservatively, but much lower rates of recurrence when wide resection is performed. Much like OKCs, they present as painless, slow-growing, bony hard masses, clinically. Radiographically, they usually present as well defined multilocular or unilocular radiolucencies.12
Non-odontogenic periapical pathologies:
Periapical cemento-osseous dysplasia (PCD) describes a benign lesion that forms when periapical bone is replaced by benign fibrous tissue containing metaplastic bone and cementum-like tissue. Classically, it presents as an incidental radiographic finding in the anterior mandible of black female patients over forty years of age.13 These lesions are most often asymptomatic and associated with vital pulps. In typical cases, there is no need for a biopsy.
Nasopalatine canal cysts occur when epithelial remnants in the nasopalatine duct are activated, either spontaneously or through developmental stimuli. Radiographically, they appear as well-corticated, round or heart shaped radiolucencies in the anterior hard palate posterior to the maxillary central incisors.14 They are generally slow-growing, painless masses which may cause divergence or resorption of adjacent tooth roots. In this case, adjacent teeth (most often #8 and #9) will display positive pulp-vitality testing.
Traumatic bone cysts lack a universally accepted etiology, but are believed to occur when trauma induced intraosseous clot formation goes awry, and leads to osteoclast mediated resorption.15 Although traumatic bone cysts share key radiologic features with true cysts, they are known as pseudocysts since they lack an epithelial lining. Classic radiographic presentation includes a unilocular, well-defined, and corticated lucency which scallops around the apices of vital teeth.16 Traumatic bone cysts are classically incidental findings in the posterior mandibles of patients under thirty years of age.16
Resorptive defects: occur due to physiologic or pathologic processes that lead to a loss of dentin, cementum, or bone.6
Internal root resorption (IRR) denotes the ongoing loss of dentin along the canal walls most often as a result of trauma, infection, or orthodontic treatment. On radiographic examination, internal resorption appears as a radiolucent round ballooning out of the root canal. IRR is most often seen in incisors and mandibular molars.17 This process is usually asymptomatic but can sometimes lead to pinkish discoloration of the crown, known as pink tooth of mummery. Ideal management of this lesion includes early detection, removal of the cause, and proper treatment. The process of internal resorption can be halted by removing blood supply to the area via conventional root canal therapy (RCT). With regard to perforating resorptive defects, an MTA plug is often utilized to create a biocompatible seal before obturation.17
The types of external root resorption (ERR) have been comprehensively addressed by Patel and Saberi (2018). In the following section I will briefly summarize the five categories of ERR covered in their paper: inflammatory, replacement, cervical, surface, and transient apical breakdown.18
- External inflammatory resorption (EIR) occurs in teeth with necrotic pulps which undergo trauma which stimulates external resorption. Radiographic findings most often include a short and ragged root surrounded by a periapical radiolucency. RCT is indicated if the tooth is deemed restorable.
- External replacement resorption (ERR) occurs as a result of extreme luxation or avulsion where the PDL cells die and are replaced with alveolar bone. Radiographic examination will reveal a loss of PDL space. There is no treatment for ERR, but affected teeth can last for many years before the root becomes completely replaced by bone.
- External cervical resorption (ECR) usually occurs due to damage to the cementum in the cervical region. Often misdiagnosed as caries, early lesions appear radiolucent with well-defined or irregular margins at the CEJ. More advanced lesions will appear mottled and may involve the pulp chamber. Treatment options include internal repair with RCT, external repair with or without RCT, replantation, periodic monitoring, or extraction.
- External surface resorption (ESR) is a transient process which will stop when the causal pressure, due commonly to orthodontic treatment, tumors, impacted teeth, and cysts, is eliminated. On radiograph, the roots adjacent to an expansile lesion will appear short, blunted, or irregular.
- Transient apical breakdown (TAB) is commonly associated with luxation trauma and features apical resorption which resolves within one year. Clinically, the tooth may have delayed or no response to pulp testing. Radiographically, the PDL space widens and the apical lamina dura is lost or blurred. In the case of TAB, imaging will return to normal within 12 months. It is critical that teeth with luxation trauma should be monitored with regular pulp testing and radiographs to determine whether a process like TAB or EIR is occurring and thus whether a RCT is indicated.
Oral squamous cell carcinoma:
The vast majority of oral cancers are squamous cell carcinomas, which originate and present in the oral mucosa, however oral cancer can rarely present as an intraosseous lesion. The annual incidence of oral squamous cell carcinoma (OSCC; not including oropharyngeal cancer) in the United States is approximately 12,000-16,000.19 Primary risk factors include smoking and heavy alcohol use. Oral lesions are often initially asymptomatic, which makes routine screening an important method for early diagnosis. Mucosal OSCC most often appears in cancer prone locations such as the soft palate, floor of the mouth, ventral and lateral tongue. Dysplastic changes often appear in these locations as red and/or white non-wipeable plaques. Findings which should raise suspicion for oral cancer include ulceration, erosion, pebbly or verrucous texture, nodularity, induration, tissue immobility, and tooth mobility. If OSCC has infiltrated into the jaws, it will present radiographically as an ill-defined radiolucency. In about half of these infiltrative cases, root resorption occurs leading to spike-type or knife-edge roots.20 Any areas suspicious for OSCC should be biopsied.
Documentation of clinical and radiographic findings:
When documenting the presence of an irregular finding in your patient’s chart or when referring them back to their general dentist or to an oral pathologist for follow up, it is helpful to include the proper descriptors. For clinical descriptions, include the location, type of lesion, shape, color, surface texture, size, borders, and any irregularities in the surrounding tissue. Types of lesions include ulcers, nodules and masses, vesicles and bulla, plaques, papules, and macules. Surface texture can, for example, be smooth, rough, irregular, velvety etc. Borders, on the other hand, are often described as sharp, ill-defined, or diffuse.
To note a radiographic finding, include the precise location, whether the lesion is localized or generalized, describe boundaries, shape, radiodensity, and any existing relationships to other structures. With regard to boundaries, a radiographic finding can be well-defined, well-corticated, ill-defined, and/or well-localized. Well-defined findings have perimeters which are easily identifiable and mark the boundary of the entire lesion. Corticated borders are radiopaque and can vary in thickness. Ill-defined findings are more diffuse with a subtle transition between normal tissue and the lesion where an exact boundary is difficult or impossible to follow. Well-localized findings are focal but lack a well-defined border. When describing radiodensity, a lesion can be opaque, lucent, or mixed. Radiopaque lesions appear lighter on film, while radiolucent lesions are dark regions on the radiograph. Finally, note the lesion’s relationship to adjacent teeth, nerve canals, and cortical bone.
Recommendations for biopsy:
The AAE advises that a biopsy be taken if enough tissue can be removed during periapical surgery, if pathology is persistent or inconsistent with endodontic disease, or if it is indicated by a patient’s medical history.21 With regard to the first indication, if there is sufficient tissue or foreign material which can be scooped or curetted out, it should be submitted for a biopsy. Lesions which cannot be identified by clinical or radiographic examination or those which have characteristics of malignancy should also be biopsied. Additionally, inflammatory changes which do not resolve as a result of endodontic treatment should be submitted for histological examination. Medical history, which suggests the utility of a biopsy, includes history of cancer or systemic osseous conditions. Routinely removing tissue for histological classification greatly increases the probability that destructive or even deadly pathologies will be identified and managed appropriately. Therefore, it is critical that endodontists are able to recognize the classic presentations of both common and atypical periapical pathology.
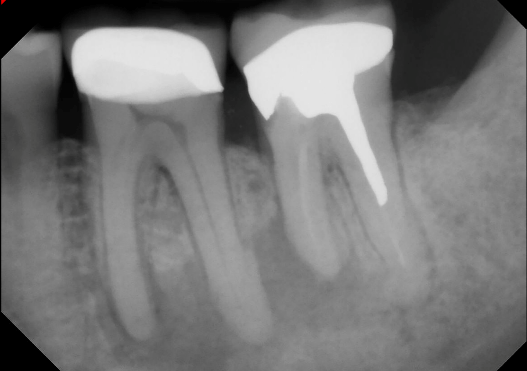
This image shows a large periapical radiolucency in the left posterior mandible which was later identified histologically as a periapical abscess.
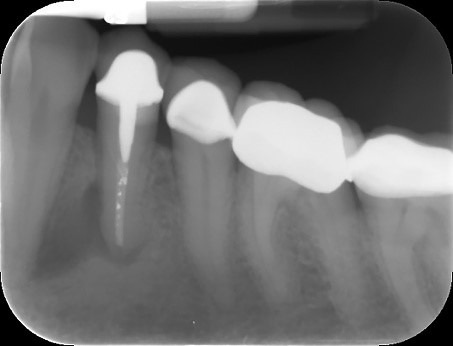
In this periapical radiograph we see a radiolucency surrounding the apex of an previously treated mandibular right premolar. Through histological examination, it was identified as a periapical cyst.
Bibliography:
- Alqaied AI, Analysis of Periapical Biopsies Submitted for Histopathological Evaluation: A Retrospective Study. Master’s Theses Paper 299. 2012. http://digitalcommons.uconn.edu/gs_theses/299
- S. Kakehashi, H.R. Stanley, R.J. Fitzgerald, The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats, Oral Surgery, Oral Medicine, Oral Pathology, Volume 20, Issue 3, 1965, Pages 340-349, ISSN 0030-4220, https://doi.org/10.1016/0030-4220(65)90166-0.
- Wiebe, S. H., M. Hafezi, H. S. Sandhu, S. M. Sims and S. J. Dixon (1996). “Osteoclast activation in inflammatory periodontal diseases.” Oral diseases 2(2): 167-180.
- Hirsch, J. M., U. Ahlstrom, P. A. Henrikson, G. Heyden and L. E. Peterson (1979). “Periapical surgery.” Int J Oral Surg 8(3): 173-185.
- Ramakrishnan L (April 2012). “Revisiting the role of the granuloma in tuberculosis”. Nat Rev Immunol. 12 (5): 352–66. doi:10.1038/nri3211. PMID 22517424.
- AAE Glossary of Endodontic Terms: Tenth Edition. 2020. https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/
- Schulz, M., T. von Arx, H. J. Altermatt and D. Bosshardt (2009). “Histology of periapical lesions obtained during apical surgery.” J Endod 35(5): 634-642
- Nair, P. N. (1998). “New perspectives on radicular cysts: do they heal?” International endodontic journal 31(3): 155-160.
- Carrillo García, Celia, Vera Sempere, Francisco, Peñarrocha Diago, Miguel, & Martí Bowen, Eva. (2007). The post-endodontic periapical lesion: Histologic and etiopathogenic aspects. Medicina Oral, Patología Oral y Cirugía Bucal (Internet), 12(8), 585-590. Recuperado en 20 de noviembre de 2020, de http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1698-69462007000800009&lng=es&tlng=en.
- R. Eversole, L. Su, S. ElMofty Benign fibro-osseous lesions of the craniofacial complex. A review Head Neck Pathol, 2 (2008), pp. 177-202
- Witmanowski H, Szychta P, Błochowiak K, Jundziłł A, Czajkowski R. Basal cell nevus syndrome (Gorlin-Goltz syndrome): genetic predisposition, clinical picture and treatment. Postepy Dermatol Alergol. 2017;34(4):381-387. doi:10.5114/ada.2017.69323
- Masthan KM, Anitha N, Krupaa J, Manikkam S. Ameloblastoma. J Pharm Bioallied Sci. 2015;7(Suppl 1):S167-S170. doi:10.4103/0975-7406.155891
- Roghi, M, Scapparone, C, Crippa, R, Silvestrini-Biavati, A, Angiero, F. (2018) “Periapical Cemento-osseous Dysplasia Clinicopathological Features.” Anticancer Research 34(5): 2533-2536
- Dedhia P, Dedhia S, Dhokar A, Desai A. Nasopalatine duct cyst. Case Rep Dent. 2013;2013:869516. doi:10.1155/2013/869516
- Xanthinaki AA, Choupis KI, Tosios K, Pagkalos VA, Papanikolaou SI. Traumatic bone cyst of the mandible of possible iatrogenic origin: a case report and brief review of the literature. Head Face Med. 2006;2:40. Published 2006 Nov 12. doi:10.1186/1746-160X-2-40
- Surej Kumar LK, Kurien N, Thaha KA. Traumatic bone cyst of mandible. J Maxillofac Oral Surg. 2015;14(2):466-469. doi:10.1007/s12663-010-0114-8
- Mittal S, Kumar T, Mittal S, Sharma J. “Internal root resorption: An endodontic challenge”: A case series. J Conserv Dent. 2014;17(6):590-593. doi:10.4103/0972-0707.144612
- Patel, S., Saberi, N. The ins and outs of root resorption. Br Dent J 224, 691–699 (2018). https://doi-org.online.uchc.edu/10.1038/sj.bdj.2018.352
- American Head & Neck Society. 2020. Oral Cavity Cancer: Professional Version – Page 3 Of 12 – American Head & Neck Society. [online] Available at: <https://www.ahns.info/resources/oral-cavity-cancer/3/> [Accessed 4 December 2020].
- Kawai, N., Wakasa, T., Asaumi, Ji. et al. A radiographic study on resorption of tooth root associated with malignant tumors. Oral Radiol. 16, 55–65 (2000). https://doi.org/10.1007/BF02492700
- Dahlkemper, P., Ang, D., Goldberg, R., Rubin, R., Schultz, G., Sheridan, B., et al. Guide to Clinical Endodontics. American Association of Endodontists, 2013.
Gabriella Blazquez is a third-year dental student at the University of Connecticut School of Dental Medicine. She has a passion for endodontics and will be applying to residencies this spring. Her dream is to teach endodontics and provide care to patients in underserved communities.
